








I’m a Nanotechnology Engineering student at the University of Waterloo. I really like this school.
Pros:
• Fantastic engineering education
• Co-op provides great experience
• Lots of opportunities to be the dumbest person in the room (surrounding yourself with high-achievers motivates you to become one)
• Campus enclosed in a ring road (feels like a little town of just students, great for the first year on-campus-living vibes)
• Attractive location (nature and green space around)
• Great public transit
Cons:
• Less party life (not necessarily a con, but party schools are nearby anyways)
• Some parts of campus aren’t very attractive
• Engineering is hard
The University of Waterloo has underground service tunnels inaccessible to students that connect every building. Once I graduate, I’ll put the map I made here.
At IQC, I used COMSOL Multiphysics software to simulate and design millimetre-wave (4-40 GHz) resonator circuits under the supervision of Dr. Brad Hauer. The goal of my project was to design resonators to be fabricated on a sapphire wafer and cooled to ~10 mK in a dilution fridge, where their quality factor can be measured.
The quality factor tells us two main things about the resonator:
A) How long it will resonate for (how much of its energy is lost as it resonates). Imagine a bell which rings out for a very long time when struck, vs one which goes “clunk” and dies off quickly. The long-ringing (high quality) bell has a high Q-factor, while the cheap clunky bell has a low Q-factor.
B) The second thing the Q-factor tells us is how narrow the frequency band is that it resonates at. A bell with a high Q-factor will ring with a very specific frequency, whereas a cheap bell with a low Q-factor will emit a wide range of frequencies (“clunk”). A crystal wine glass vibrates and eventually shatters when enough resonant energy builds up — a sign of a very high Q.
High frequency selectivity (i.e. high Q-factor) is essential for quantum circuits. If resonators overlap in frequency, they interfere with each other, introducing noise and corrupting the tiny signals we’re trying to measure. Resonators with high Q-factor can also store information and remain “coherent” for longer periods of time (think: bell ringing for a long time).
Most superconducting resonators are in the 4-8 GHz range, so our research on high frequency 4-40 GHz resonators provides overlap — to compare our data to existing data — while also probing underexplored territory. One of the key advantages of higher-frequency resonators is their much smaller footprint, which scales down with increasing frequency.
The resonators I designed were LC-circuits, meaning they had an inductor component and a capacitor component. The inductor component stored energy in a magnetic field, while the capacitor component stored energy in an electric field. The resonance occurred as energy oscillated between these two components.
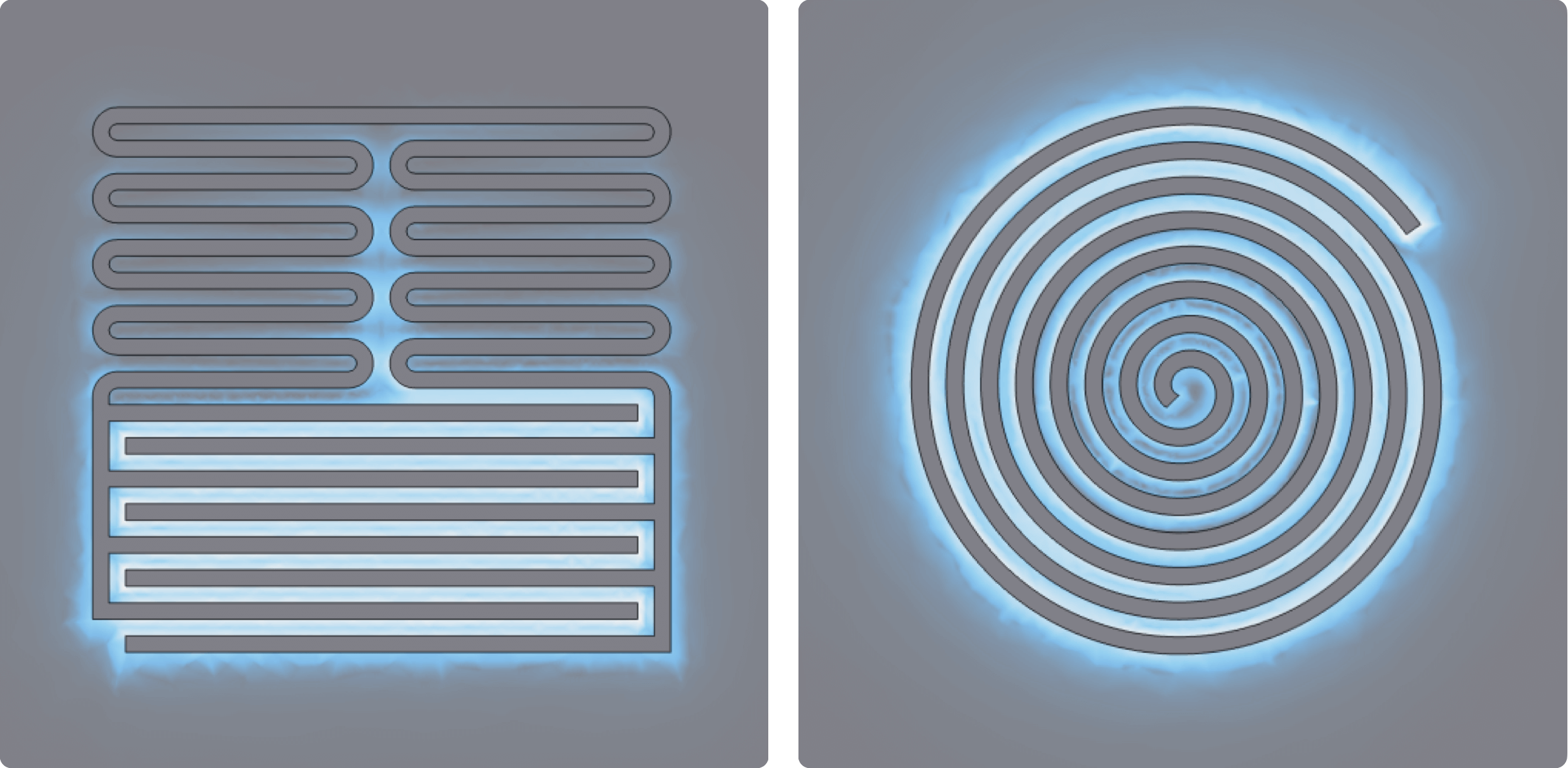
I worked with two resonator designs. The first is a lumped element design, where there is a meandering inductor at the top and an interdigitated capacitor at the bottom, and the second is a spiral resonator, which blends both elements together into a more compact shape.
My simulations helped predict resonant frequencies and how to achieve various coupling rates, which is helpful for an accurate measurement of the quality factor.
At mySolar, I wore many hats. My days consisted mostly of glorified IT work. The central CRM system that the company ran off of was a work in progress, so I was in weekly communication with the CEO of that company (Sunbase). I created custom email templates to customers using HTML and CSS, which I had learned only weeks prior while making this website. I managed several requests per day, every day, to keep things running smoothly for everyone from customers to my fellow coworkers behind the scenes.
I learned the ins and outs of residential solar operations. We did not manufacture the panels, so most of the technical knowledge I acquired was business-related rather than science-related.
In January 2023, I started applying for my first engineering co-op. Four months and 700 applications later, I was still unemployed and losing hope. I ended up taking a job as a door-to-door salesman, selling pest control. The job was 100% commission-based, meaning that if I made no sales, I made no money. My parents were terrified for me and gave me two weeks to make a reasonable amount of money, or I’d have to find something else that paid by the hour (which was great advice).
Turns out, the sales gig worked out. I made two sales on my first day (earning $400), and then did the same thing again on my second day, and my third. I had a successful summer, closing around 140 accounts and making over $25k, doubling what I would’ve made in an engineering co-op position.
The key to my success was consistency. In the office, they literally called me “Mr. Consistent,” because while some reps could occasionally throw up 5 or 6 sales in a day, I had a much narrower distribution of about 2 per day and almost never came home empty-handed. I knocked in rain, shine, or forest-fire smoke. I kept a good attitude, which wasn’t hard, because the majority of people are just nice. Staying resilient and not caring if people decided to buy was crucial, because while I made 140 sales, I also faced around 9,000 rejections.
I always spoke to people about anything interesting of theirs that I could see (cars, pianos, etc.), not only to build rapport for the sale but also because I was genuinely interested. This led to some amazing experiences: a ride in someone’s Model 3 Performance, another guy’s Cadillac CT5-V Blackwing (dream car), a crash course on how one guy built his first Shelby Cobra kit car, and what he was doing differently on his second one (which was on a lift in his garage). I also got to play a lady’s Steinway grand piano in her living room, which was incredible, and gave me the revelation that I actually prefer Yamahas.
One of the most incredible parts of the summer was what happened after knocking was done. Myself and 80 other top sellers in the company were taken to an all-inclusive resort in Cuba. Being amongst such a huge group of the most socially outgoing and driven people you’ll ever meet, and being recognized as the guy who outsold everyone in his territory, including his manager, made for one of the coolest, most rewarding vacations I think I’ll ever have. The CEO of the company, Brendan, spoke to me in Cuba about whether I would come back to knock in the future. I told him that engineering was where I wanted to be, and he ended up hooking me up with a solar company he had recently become a co-owner of. Thanks to that connection, I didn’t need to submit a single application to get my next co-op, which was a wonderful change of pace from the previous term.
To anyone considering an opportunity like this: I do not recommend it to the majority of people. Of the 400 or so rookie sales reps that worked the same summer as me, roughly three quarters of them made less than minimum wage. Even though I did relatively well, it was the most stressful job I’ve ever worked, and I chose to not go back (although most who made above minimum wage did go back). If you are considering a job like this, you need to be very confident in your social skills.
Best Buy was probably the most fun job I’ve ever had. Helping people, interacting with strangers, and doing physical tasks made work so enjoyable. Everyone I worked with also made work so good. It was a wonderful group of people.
When I was first hired, I was 14 years old, and I worked my first shift on Black Friday. I worked two weeks, and then the managers found out when they were putting me into their system to pay me that company policy stated that they weren’t allowed to hire anyone under 15. I was given an envelope of cash and told to come back in a year. I did.
While working there, I created a short program to run on my phone in Apple Shortcuts, which helped speed up the money counting at the end of each day.
There’s something about working a low-stress position, interacting with strangers, that I found incredibly fulfilling, despite the low pay. My sister can attest to this. Hazel spent high school working at a local coffee shop, and she absolutely loved it. I hope to work more jobs in my life that require me to regularly meet new people.
I planned this website as a two-week project (as a resume booster), after Elai Mizrahi and Eshaan Mehta showed me the websites they had made for themselves.
I didn’t use a boilerplate to make this site. I knew literally nothing about web design, so I found it easier to start from complete scratch (any existing code was very intimidating). Initially, I would come up with ideas and ChatGPT would translate them into code, but as things got more complicated, ChatGPT struggled to keep up. Luckily, by that point I had accidentally learned a decent amount of HTML and CSS, and W3Schools filled in any other gaps.
During any of the little coding projects I had done in the past, the aspect that I enjoyed most was creating an elegant user experience. Web design, being almost entirely user experience, ended up being the most fun project I had done yet; it stopped being a two-week resume booster project and became a four-month passion project.
I use Vercel for the hosting of the site. I highly recommend using them as your web hosting platform if you decide to take on a project like this. This whole website costs me around $15 a year for the domain “keogan.ca,” which I got from GoDaddy; the hosting via Vercel is completely free.
My high school operated on a 2-day schedule system. Day 1s were on odd dates, and day 2s were on even dates. On a day 1, classes were in the order A-B-C-D, and on day 2s, classes were in the order B-A-D-C. It was just annoying enough to warrant me creating a schedule program that answered the question of what class you have with the click of a button on your phone.
I used the iPhone Shortcuts app. When you clicked the button on your home screen to run the shortcut, it would quickly display which class you had next, and how long until it starts. If you ran the program while already in a class, it would display how long it would be until the class ended.
I created a website for it, and created working versions for 20 high schools in Ottawa. It never ended up taking off beyond my school, but it was fun to make and the people around me seemed to appreciate it.

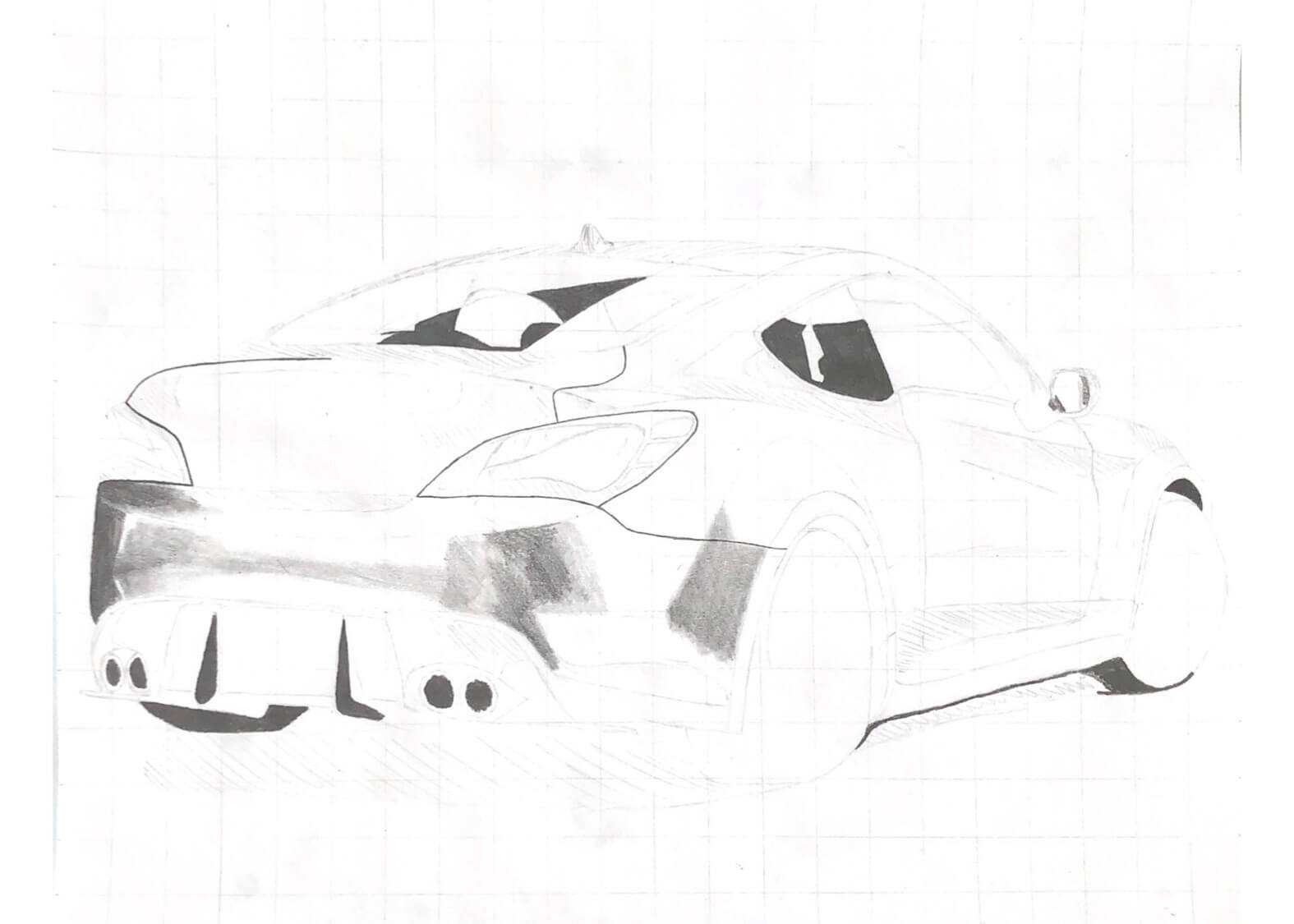
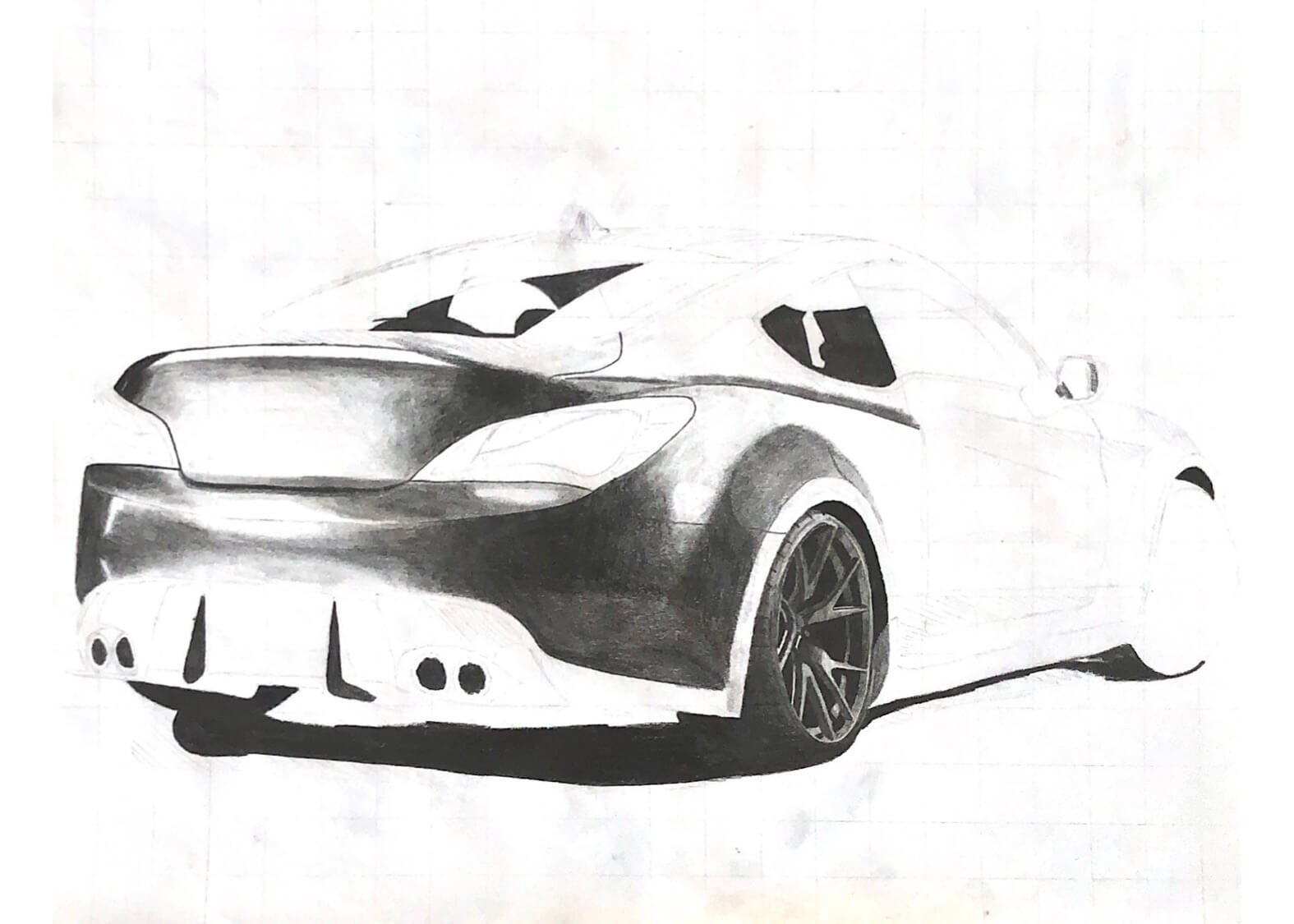
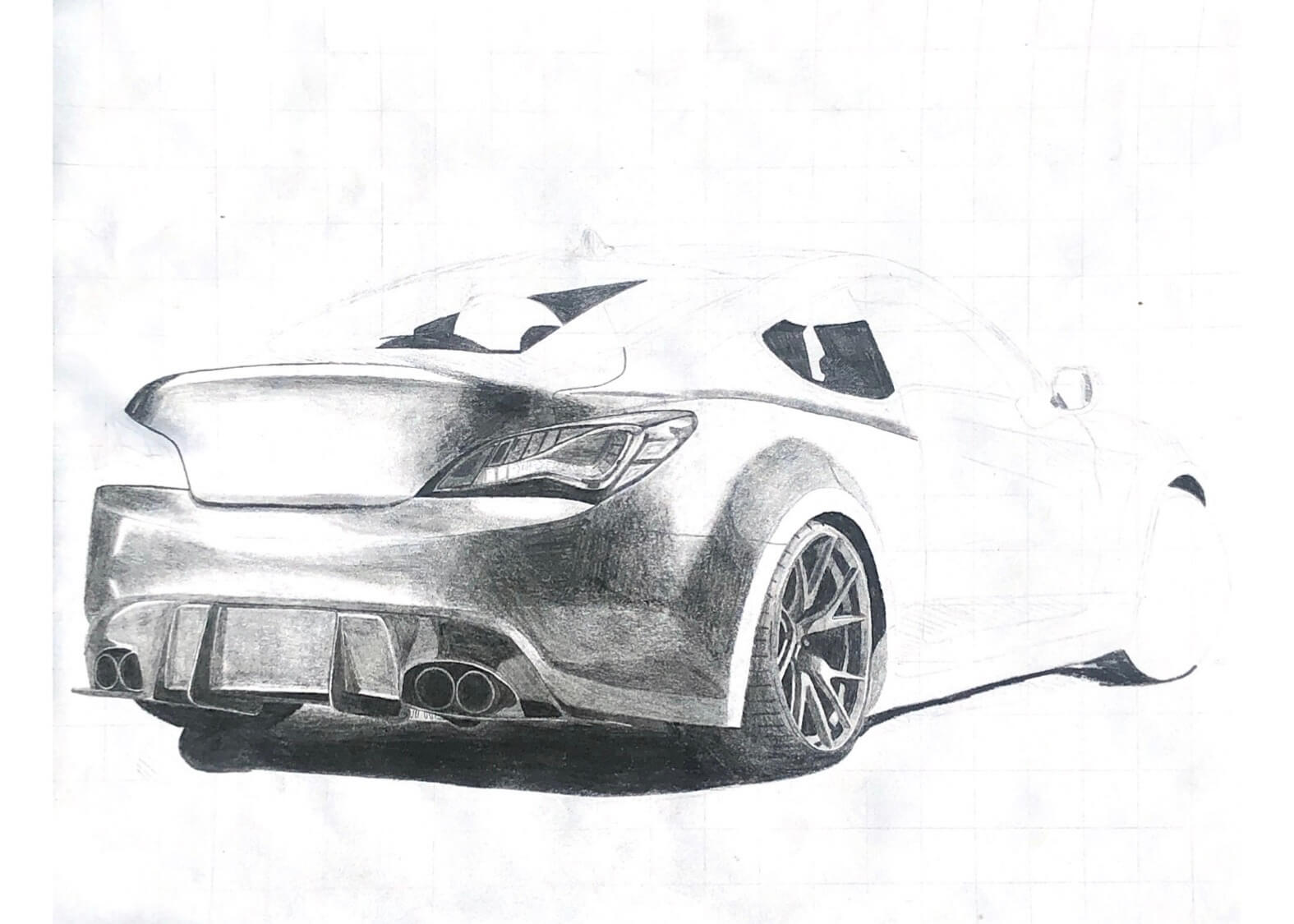
One of the first hobbies I ever had was drawing cars. The earliest thing I remember wanting to be when I grew up was a car designer. I loved sketching and drawing side profiles of imaginary vehicles. In grade 7, we had an art project of any style that we wanted. I chose a hyperrealistic pencil drawing of a Mustang, which introduced me to an entirely new passion.
My hyperrealism drawings are not from my imagination; they are real photographs which I do my best to recreate. To maintain proportions in my drawings, I overlay a grid on my reference photo, and the first thing I draw on the paper is a matching grid. From there, I lightly draw the prominent lines in the drawing with an extremely hard pencil (5H), and add darker and darker layers.
At some point, I would like to experiment with placing a display or printed photo underneath my paper, projecting the image across, and then tracing it, for more accurate realism.


Everything in the universe can be measured using the seven fundamental SI units, or their derivatives. All other units of measurement can be reduced down to these seven units, which create a consistent framework for all of science. Each unit corresponds to a specific physical property:
1. meter (m) – length
2. kilogram (kg) – mass
3. second (s) – time
4. ampere (A) – electric current
5. kelvin (K) – temperature
6. mole (mol) – amount of substance
7. candela (cd) – luminous intensity
Knowing that our universe in its entirety can be represented using only these units begs some curiosity over what the largest and smallest values of these units are in our universe. To use length as an example, the largest measurable thing is the universe itself, at around 9 × 1026 meters in diameter. That’s 9, followed by 26 zeros, or 900,000,000,000,000,000,000,000,000 meters. The smallest measurable thing in our universe is a particle called an upsilon meson. It has a diameter of roughly one femtometer, or 10-16 (0.000,000,000,000,001) meters. An upsilon meson is composed of a bottom quark and an anti-bottom quark, and will require a small lesson on the Standard Model to explain.
It is common knowledge that everything that we can touch is made up of atoms, which are made up of protons, neutrons, and electrons. In the late 20th century, it was discovered that protons and neutrons can be broken down into smaller components, called quarks. Quarks are one of the many elementary particles in our universe. These elementary particles are arranged as the Standard Model, which groups them into quarks, leptons, and bosons. We don’t interact with the majority of these particles; the important ones to our reality are the up and down quarks, the electron, and the photon. Protons are made up of 2 up quarks and 1 down quark, while neutrons are composed of 2 down quarks and 1 up quark.
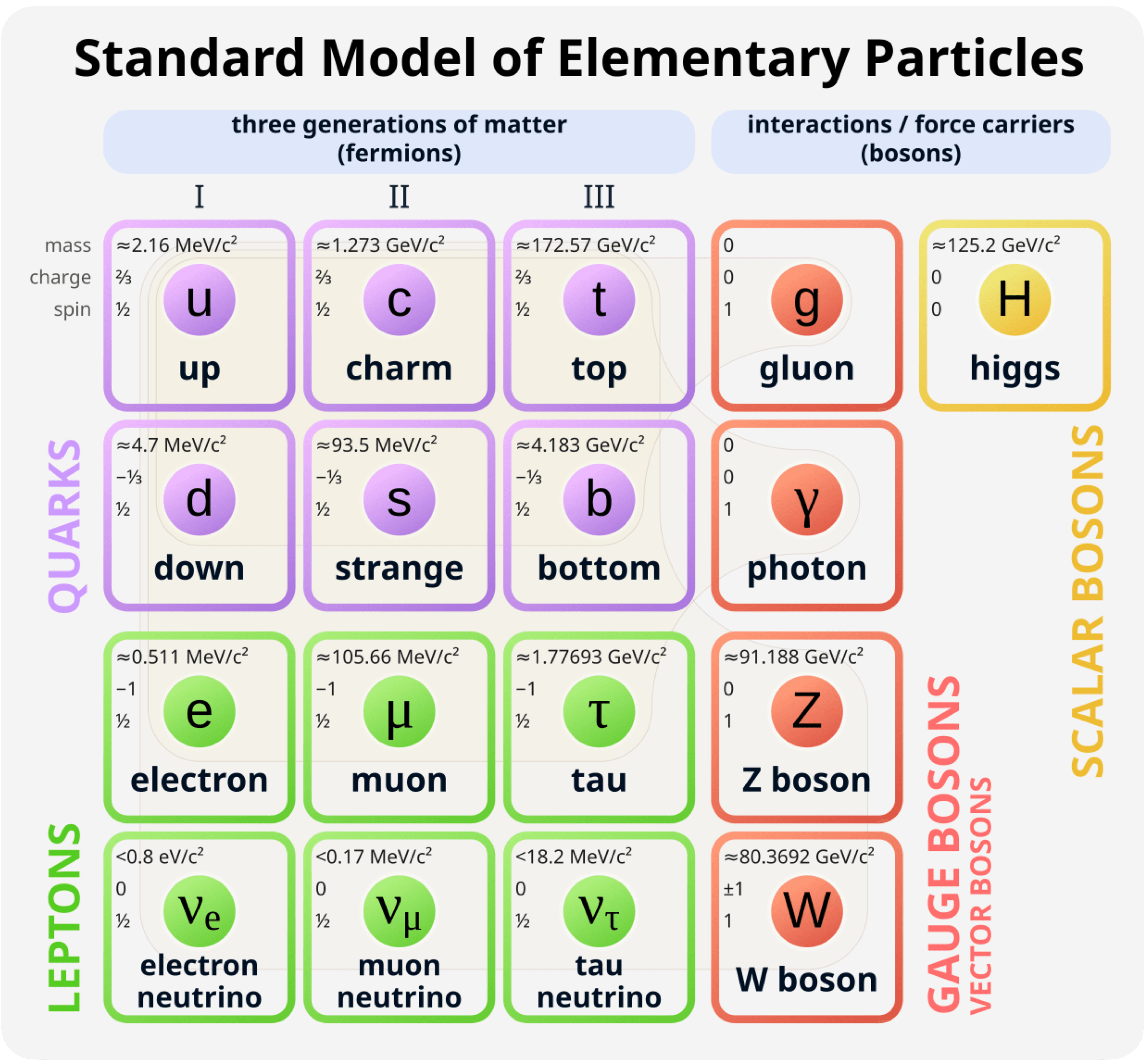
You may notice that the particle I said to be the smallest in the universe, the upsilon meson, is not in the Standard Model. As it turns out, the elementary particles found in the Standard Model don’t have any measurable shape or size. They are effectively zero-dimensional. The concept of length only arises through composite particles, ie. combining two or more of these elementary particles together to form new particles such as protons or neutrons. When initially researching this, it appeared that protons were the smallest measurable thing in our universe, but that conclusion seemed flawed, since protons were made up of 3 quarks. What about a particle made up of 2 quarks?
2-quark particles can exist in the form of mesons. You may have heard of antimatter, which is made of particles which have opposite charge, but are equal in all other regards to the normal particles that make up nearly all of our universe. 2-quark mesons are made up of one quark and one anti-quark. So as it turns out, the smallest measurable thing in our universe is a 2-quark meson called an upsilon meson. The reason we don’t ever hear about upsilon mesons, or any other composite particles aside from protons or neutrons is because they are incredibly unstable. An upsilon meson has a mean lifetime of around 10-20 seconds before decaying, whereas the decay of a proton has never been observed (meaning they may be the only perfectly stable particle. While neutrons within the nucleus of an atom are completely stable as well, outside of a nucleus, neutrons have a lifetime of only 15 minutes before they decay into a proton, electron, and anti-neutrino).
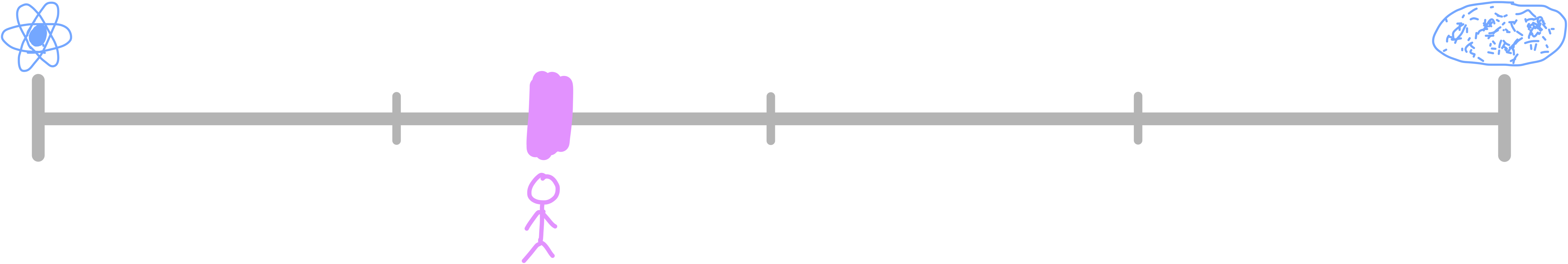
So now knowing what the largest and smallest items are, how do humans stack up? Where have we been placed in the universe, on the scale of length? This can be easily shown with a logarithmic scale.
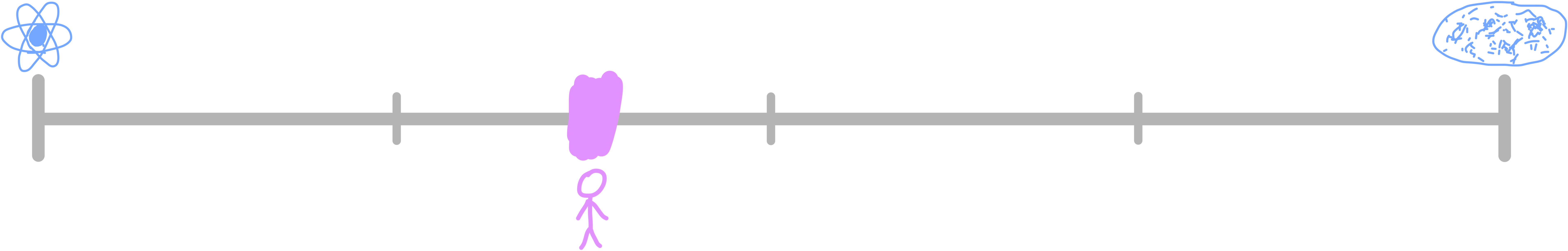
We’re about 38% of the way across. I’m not sure why this information is so satisfying, but it’s cool to know where we sit. How do we fare on the scale of mass?
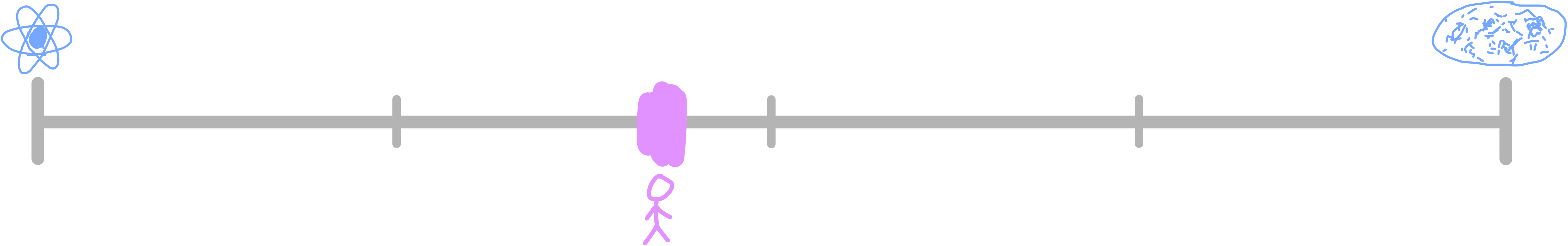
The mass of the universe has been calculated to be approximately 1053 kg. The smallest mass that we know of is the electron neutrino, which you can find in the Standard Model we looked at earlier. It has a mass of 10-37 kg. Similarly to our conversation about the smallest measurable thing in the universe by length, there are particles which have zero mass, like the photon, but for the same reasons we didn’t use photons or electrons (zero length particles) as our length limit, we won’t use photons or other bosons (zero mass particles) as our mass limit. Between the mass of electron neutrinos and the universe, we’re slightly closer to the neutrinos, at 43% of the way across.
The universe has been around for 13.8 billion years, which is 4 × 1017 seconds. The smallest amount of time could be described as the smallest process which can occur, according to our other limits. In this case it would be the time it takes for a photon travelling at the speed of light to cross our smallest distance: the diameter of an upsilon meson. This is around 3 zeptoseconds, or 3 × 10-24 seconds. The human value for time is more difficult than for length or mass. It’s not as clear what value should be used. I’ll include three different values in the scale, so you can decide which one is most important to you.
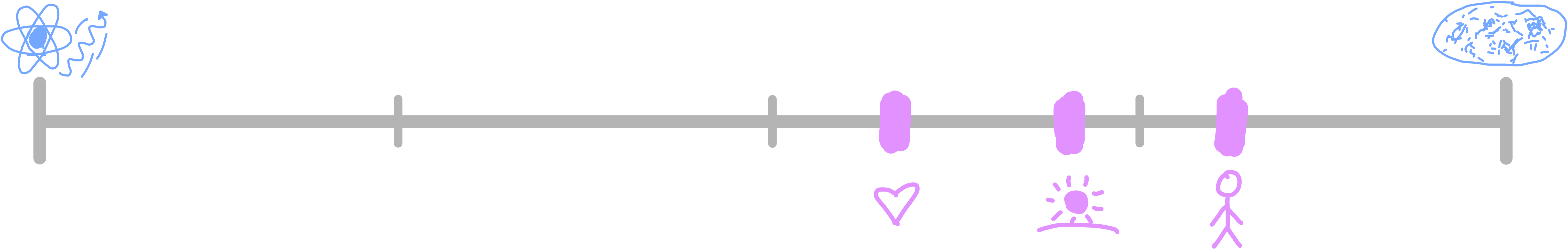
The first is one second, which corresponds to one human heartbeat. That’s 58% of the way across. Next is one day (86,400 seconds), since we structure our lives around daily cycles. That’s 70%. The final one is the average human lifespan of 73 years, which is 81% of the way across. If I got to choose one of these units to experience the largest amount of, I would pick time. I think it’s beautiful that we’re so far across this scale.
The research for this scale has been problematic. For starters, it’s much less intuitive, since (if we’re lucky) most of us never have a memorable quantitative experience with electric current, but maybe we could use the value of current in our brains (10-12 A), or in our nerves/muscles (10-3 to 10-6 A). The largest ampere value ever recorded was 1018 A, from a cosmic jet two billion light years away. This value is fine, but it was recorded in 2011, and there have probably been larger currents in the past and there will probably be larger currents in the future, so it’s not a very consistent or meaningful value, unlike what we have for other units.
The other issue is that the lower bound is not clear at all. Electric current measures charge (electrons) per second. Compared to other units, this is arbitrary, useful only to humans because biological systems and the systems they create often require electricity. Maybe the lowest bound could be one electron per second, but then why not decrease that to one electron every two seconds, and so on? Unfortunately, this unit doesn’t make much sense to address.
Temperature is an interesting unit because the lower bound is a little foggy as well. Technically, there is a hard lower bound of 0 kelvin, but the laws of thermodynamics and quantum mechanics make it impossible for anything to reach 0 K. This is good news for us because a value of 0 would not compute in our logarithmic scale. Throughout the entire universe, space itself is bathed in 2.725 K radiation from the Big Bang, so nothing naturally cools below that without external intervention.
That’s not quite the minimum though, because sometimes there is external intervention. The coldest naturally occurring temperature is found in the Boomerang Nebula, which is expanding extremely fast, causing adiabatic cooling (similar to how expanding gas in a spray can gets cold). It reaches around 1 K.
This, however, is still not the lowest bound. Humans have accomplished something truly incredible. In 2021, scientists achieved a temperature of 38 picokelvin (3.8 × 10-11 K). This means that as far as we know, humans have created the coldest temperatures in the entire history of the universe - by eleven orders of magnitude. The only thing that could beat us is an alien civilization with even more advanced cooling tech. That’s pretty cool.
For the sake of this discussion, we’ll use the naturally occurring 1 K of the boomerang nebula as our lowest value, since the man-made value is constantly undergoing new records and is less meaningful in the context of the universe. The highest temperature the universe has ever experienced was shortly after its inception, when it reached 1032 K. This temperature is so high that our laws of physics break down for anything hotter than this. At 310 K, humans are only 8% of the way across this scale.
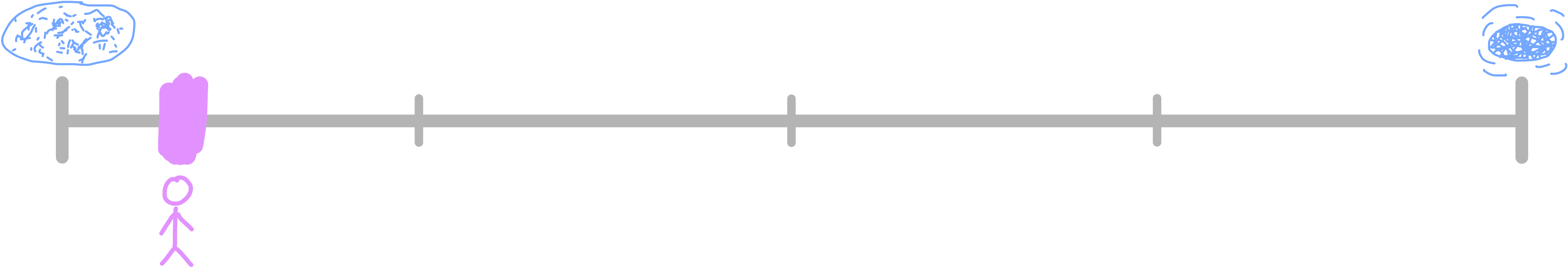
This scale is pretty similar to the one for mass, since generally, the more particles you have, the more massive you are, but let’s check it out anyways. Amount of substance is measured in moles, which is defined as 6.022 × 1023 items. We’ll use atoms as our items. The logical lower bound is 1 atom, which is 1 divided by Avogadro’s constant, which comes out to 1.7 × 10-24 moles. A typical human has around 11,600 moles of atoms, and the universe is estimated to have roughly 1056 moles of atoms. Humans are 35% of the way across this scale.
The reason that this scale does not align perfectly with the mass scale is because the average atomic mass of the atoms in a human body is much higher than the average atomic mass of the atoms in the universe. Most of the universe is made up of hydrogen (~92% of all atoms), which has an atomic mass of just 1 u. Helium makes up most of the rest, at 4 u. These are the lightest elements, so the universe’s average atomic mass is skewed very low—around 1.3 u per atom.
Compare that to the human body, which is mostly oxygen, carbon, hydrogen, nitrogen, calcium, and phosphorus. While hydrogen is still present, it’s vastly outnumbered by oxygen (16 u), carbon (12 u), and the other heavier elements. The average atomic mass of a human body is closer to 12 u per atom.
Candela is another one of those units that is very human-centric. Unlike total power output (which would be measured in watts), the brightness measured by candelas accounts for how the human eye perceives different wavelengths of light. Similarly to the ampere, candelas measure something per second, so the lower bound for this unit is unclear, because while we could choose one photon per second, we could also choose one photon every two seconds, etc.
To make matters worse, the upper bound is just as unclear. Determining luminous intensity requires precise knowledge of the angular distribution of light, which has so far not been possible for the brightest items in our universe, such as supernovae and quasars. They’re too far away for us to conduct an analysis of their light like this.
All of this information is up for your own interpretation. There’s beauty to it all, and gives lots of room for deep thoughts. How would life be different if we were at different places in each of these scales? How do different scales affect each other? Why is our position in each of these scales such perfect sweet spots? If aliens existed out there, could they exist in vastly different places within the scales?
I hope you enjoyed learning about all this as much as I did!
Always Remember Us This Way: Tutorial
O Canada: Tutorial Coming Soon
Skyfall: Tutorial
Take Me To Church: Tutorial, Sheet Music
Thanks to Jakob Focke for contributing to have the Take Me To Church sheet music made, and to Victor Rondon for transposing it! For sheet music for other covers, email me at keog@nlara.de and we can work something out! I am always willing to supply my MIDI files.
My early covers were usually learned with a combination of sheet music and by ear, but with time that balance has shifted more and more towards learning by ear, often kick-started with Guitar Tabs to learn the chords.
I was classically trained, so sheet music was the basis of my weekly lessons from ages 4 through 14. Around the age of 12, I discovered Costantino Carrara’s piano covers on YouTube, which inspired me to start messing around with pop covers too. Over the next few years I found that adding my own flourishes and creative touches to the arrangements came more naturally when learning by ear, and the songs also required no additional effort to memorize.
My covers are recorded in two parts. The audio is recorded in advance, using a Casio CDP-S350 keyboard wired through Logic Pro X. I use a digital piano sound from Garritan called CFX Lite, which uses the sound of the Yamaha CFX Grand piano. The video is recorded later using multiple shots.